Avogadro’s Contributions to Science In the early 1800s, scientists’ ideas about the particles we now call atoms and molecules were very limited and often incorrect. Avogadro was deeply interested in finding out how the basic particles of matter behave and come together to form chemical compounds. He studied the work of two other scientists.
- As a tribute to his contributions to molecular theory, a number was named after him. It is known as Avogadro’s number or constant, and has the value of 6.0221367×10 23 Regarded as the ‘father of modern chemistry’, Antoine Lavoisier established the law of conservation of masses.
- Avogadro's Law is one of the most important laws to gas chemistry, but it wasn't always viewed this way. In this lesson we will learn how Amedeo Avogadro came up with this law and how it is used.
- Avogadro began experimenting with electricity, for which there was a strong tradition at the University of Turin. It is not likely to be a coincidence that this was at just the time that European.
- Avogadro's number, or Avogadro's constant, is the number of particles found in one mole of a substance. It is the number of atoms in exactly 12 grams of carbon-12.This experimentally determined value is approximately 6.0221 x 10 23 particles per mole.
Avogadro's hypotheses
Amedeo Avogadro's principal contribution to chemistry was a paper in which he advanced two hypotheses: (1) that equal volumes of gas contain equal numbers of molecules and (2) that elementary gases such as hydrogen, nitrogen, and oxygen were composed of two atoms.[1] For simplicity, let us call the first the 'volumes' hypothesis and the second the 'diatomics' hypothesis. Avogadro was correct in both of these hypotheses, which he used to reconcile and correct Dalton's atomic hypothesis with Gay-Lussac's results on combining volumes.1) If the volumes hypothesis is correct, then the relative masses of gas molecules can be computed from gas densities. (After all, the density is the mass of a unit volume, and that volume contains the same number of molecules, whatever the gas.) Construct a table of relative molecular masses based on the gas densities reported by Avogadro and using hydrogen as the unit molecular mass.
| molecule | gas density (air = 1) |
|---|---|
| oxygen | 1.10359 |
| hydrogen | 0.07321 |
| nitrogen | 0.96913 |
| oxymuriatic acid | 2.470 |
| water vapor | 0.625 |
| nitrous gas | 1.03636 |
| nitrous oxide | 1.52092 |
| muriatic acid gas | 1.278 |
2) The mass scale computed in the previous exercise has the hydrogen molecule as its unit mass. It might make more sense to take the hydrogen atom as the unit. What is the mass of the hydrogen molecule if the hydrogen atom is one mass unit? On this sacle, what is the mass of the oxygen molecule, nitrous gas, etc.?
3) Hydrogen, oxygen, nitrogen, and 'oxymuriatic acid' are all diatomic elements. (Nowadays we call 'oxymuriatic acid' chlorine.) On this scale where a hydrogen atom is one mass unit, what are the atomic masses of these elements?
4) Assume that molecules are made of small whole numbers of atoms. The masses of all of the constituent atoms ought to add up to the mass of a molecule.
a) Water vapor is a compound of hydrogen and oxygen. How many atoms of each element in one molecule of water vapor? Show that the molecular mass of water vapor is consistent with the atomic masses of the atoms and your proposed formula. (For example, Dalton thought that the formula for water was HO. If that were correct, then the molecular mass of water vapor ought to equal the atomic mass of hydrogen plus that of oxygen.)
b) Nitrous gas is a compound of nitrogen and oxygen. How many atoms of each element in one molecule of nitrous gas? Show that the molecular mass is consistent with the formula.
c) Nitrous oxide is another compound of nitrogen and oxygen. How many atoms of each element in one molecule of nitrous oxide? Show that the molecular mass is consistent with the formula.
d) Muriatic acid gas is a compound of hydrogen and oxymuriatic acid. How many atoms of each element in one molecule of muriatic acid gas? Show that the molecular mass is consistent with the formula.
5) Write balanced chemical equations for the formation of water vapor, nitrous gas, nitrous oxide, and muriatic acid from their diatomic elements.
Reference
Amedeo Avogadro, 'Essay on a Manner of Determining the Relative Masses of the Elementary Molecules of Bodies, and the Proportions in Which They Enter into These Compounds,' Journal de Physique73, 58-76 (1811) [1]Actually, Avogadro proposed that these gases were composed of two parts or a multiple of two. Copyright 2003 by Carmen Giunta. Permission is granted to reproduce for non-commercial educational purposes.| Back to the Classic Calculations home page |
| Back to the top of the Classic Chemistry site |
Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto,[1] Count of Quaregna and Cerreto (9 August 1776, Turin, Piedmont – 9 July 1856) was an Italian savant. He is most noted for his contributions to molecular theory, including what is known as Avogadro's law. In tribute to him, the number of elementary entities (atoms, molecules, ions or other particles) in 1 mole of a substance, 6.02214179(30)×1023, is known as the Avogadro constant.
Amedeo Avogadro
Biography
Amedeo Carlo Avogadro was born in Turin, Italy in 1776 to a noble family of Piedmont, Italy.
He graduated in ecclesiastical law at the early age of 20 and began to practice. Soon after, he dedicated himself to physics and mathematics (then called positive philosophy), and in 1809 started teaching them at a liceo (high school) in Vercelli, where his family had property.
In 1811, he published an article with the title Essai d'une manière de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons ('Essay on Determining the Relative Masses of the Elementary Molecules of Bodies and the Proportions by Which They Enter These Combinations'), which contains Avogadro's hypothesis. Avogadro submitted this essay to a French journal, De Lamétherie's Journal de Physique, de Chimie et d'Histoire naturelle (Journal of Physics, Chemistry and Natural History) so it was written in French, not Italian. (Note: In 1811, northern Italy was under the rule of the French Emperor Napoléon Bonaparte.)
In 1820, he became professor of physics at the University of Turin. After the downfall of Napoléon in 1815, northern Italy came under control of this kingdom.
He was active in the revolutionary movements of 1821 against the king of Sardinia (who became ruler of Piedmont with Turin as his capital). As a result, he lost his chair in 1823 (or the university officially declared, it was 'very glad to allow this interesting scientist to take a rest from heavy teaching duties, in order to be able to give better attention to his researches').[2]
Eventually, Charles Albert granted a Constitution (Statuto Albertino) in 1848. Well before this, Avogadro had been recalled to the university in Turin in 1833, where he taught for another twenty years.
Little is known about Avogadro's private life, which appears to have been sober and religious. He married Felicita Mazzé and had six children.
Some historians suggest that he sponsored some Sardinian revolutionaries, who were stopped by the announcement of Charles Albert's constitution.
Avogadro held posts dealing with statistics, meteorology, and weights and measures (he introduced the metric system into Piedmont) and was a member of the Royal Superior Council on Public Instruction.
In honor of Avogadro's contributions to molecular theory, the number of molecules in one mole was named Avogadro's number, NA or 'Avogadro's constant'. It is approximately 6.0221415 × 1023. Avogadro's number is used to compute the results of chemical reactions. It allows chemists to determine amounts of substances produced in a given reaction to a great degree of accuracy.
Johann Josef Loschmidt first calculated the value of Avogadro's number, often referred to as the Loschmidt number in German-speaking countries (Loschmidt constant now has another meaning).
Accomplishments
Avogadro's Law states that the relationship between the masses of the same volume of different gases (at the same temperature and pressure) corresponds to the relationship between their respective molecular weights. Hence, the relative molecular mass of a gas can be calculated from the mass of sample of known volume.
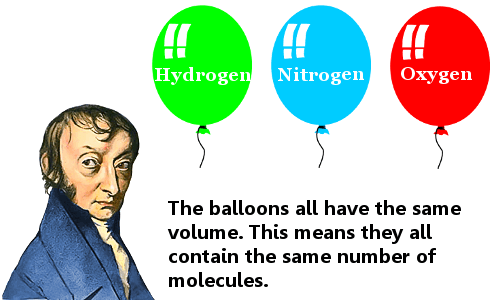
Avogadro developed this hypothesis after Joseph Louis Gay-Lussac had published in 1808 his law on volumes (and combining gases). The greatest problem Avogadro had to resolve was the confusion at that time regarding atoms and molecules. One of his most important contributions was clearly distinguishing one from the other, stating that gases are composed of molecules, and these molecules are composed of atoms. For instance, John Dalton did not consider this possibility. Avogadro did not actually use the word 'atom' as the words 'atom' and 'molecule' were used almost without difference. He believed that there were three kinds of 'molecules,' including an 'elementary molecule' (our 'atom'). Also, more attention was given to the definition of mass, as distinguished from weight.
In 1815, he published Mémoire sur les masses relatives des molécules des corps simples, ou densités présumées de leur gaz, et sur la constitution de quelques-uns de leur composés, pour servir de suite à l'Essai sur le même sujet, publié dans le Journal de Physique, juillet 1811 ('Note on the Relative Masses of Elementary Molecules, or Suggested Densities of Their Gases, and on the Constituents of Some of Their Compounds, As a Follow-up to the Essay on the Same Subject, Published in the Journal of Physics, July 1811') ([1]), about gas densities.
In 1821 he published another paper, Nouvelles considérations sur la théorie des proportions déterminées dans les combinaisons, et sur la détermination des masses des molécules des corps (New Considerations on the Theory of Proportions Determined in Combinations, and on Determination of the Masses of Atoms) and shortly afterwards, Mémoire sur la manière de ramener les composès organiques aux lois ordinaires des proportions déterminées (Note on the Manner of Finding the Organic Composition by the Ordinary Laws of Determined Proportions).
In 1841, he published his work in Fisica dei corpi ponderabili, ossia Trattato della costituzione materiale de' corpi, 4 volumes.
Response to the theory
The scientific community did not give great attention to his theory, so Avogadro's hypothesis was not immediately accepted. André-Marie Ampère achieved the same results three years later by another method (in his Sur la détermination des proportions dans lesquelles les corps se combinent d'après le nombre et la disposition respective des molécules dont leurs particules intégrantes sont composées -- On the Determination of Proportions in which Bodies Combine According to the Number and the Respective Disposition of the Molecules by Which Their Integral Particles Are Made), but the same indifference was shown to his theory as well.
Only through studies by Charles Frédéric Gerhardt and Auguste Laurent on organic chemistry was it possible to demonstrate that Avogadro's law explained why the same quantities of molecules in a gas have the same volume.
Unfortunately, related experiments with some inorganic substances showed seeming exceptions to the law. This was finally resolved by Stanislao Cannizzaro, as announced at Karlsruhe Congress in 1860, four years after Avogadro's death. He explained that these exceptions were due to molecular dissociations at certain temperatures, and that Avogadro's law determined not only molecular masses, but atomic masses as well.
Amedeo Avogadro Contribution To Chemistry
In 1911, a meeting in Turin commemorated the hundredth anniversary of the publication of Avogadro's classic 1811 paper. King Victor Emmanuel III attended. Thus, Avogadro's great contribution to chemistry was recognized.
Rudolf Clausius, with his kinetic theory on gases, gave another confirmation of Avogadro's Law. Jacobus Henricus van 't Hoff showed that Avogadro's theory also held in dilute solutions.
Avogadro is hailed as a founder of the atomic-molecular theory.
See also
Wikisource has the text of the 1911 Encyclopædia Britannica article Avogadro, Amedeo, Conte di Quaregna.
Avogadro (lunar crater)
Avogadro's constant
References
What Is Avogadro In Chemistry
^ Guareschi, Icilio (1911), 'Amedeo Avogadro e la sua opera scientifica', Opere scelte di Amedeo Avogadro, Turin: Accademia delle scienze, pp. i–cxl.
^ Link to page at the University of Piedmont in Italy
Further reading
Avogadro Contribution To Chemistry
Hinshelwood, C. N.; Pauling, L. (1956), 'Amedeo Avogadro', Science 124 (3225): 708–713, 1956 Oct 19, doi:10.1126/science.124.3225.708, PMID 17757602
Cavanna, D. (1956), 'Centenary of the death of Amedeo Avogadro', Minerva farmaceutica 5 (6): 134–7, 1956 Jun, PMID 13369233
Crosland, M. P. (1970), Avogadro, Amedeo, 1, New York: Charles Scribner's Sons, pp. 343–350, ISBN 0-684-10114-9.
Morselli, Mario. (1984). Amedeo Avogadro, a Scientific Biography. Kluwer. ISBN 90-277-1624-2.
Review of Morselli's book: Pierson, S. (1984), 'Avogadro and His Work: Amedeo Avogadro', Science 226 (4673): 432–433, 1984 Oct 26, doi:10.1126/science.226.4673.432, PMID 17799933
Retrieved from 'http://en.wikipedia.org/'
All text is available under the terms of the GNU Free Documentation License
